Sulfates
Chemistry
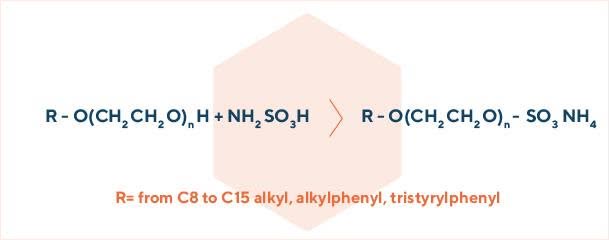
In this class of surfactants, the anionic group is linked to the molecule through an oxygen-sulfur bond.

We manage two different sulfation processes: with sulfamic acid and with chlorosulfonic acid. In both cases the starting substrate is an already ethoxylated intermediate (alcohol ethoxylates, alkylphenol ethoxylates, tristyrylphenol ethoxylates, etc.).
In the sulfamic acid process, the sulfate ammonium salt is directly obtained. In the chlorosulfonic acid process, the intermediate acid sulfate is immediately neutralized with sodium hydroxide, to obtain the sulfate sodium salt.
Being derived from an ethoxylated intermediate, our sulphate surfactants carry both, a polyoxyethylene chain and an anionic group. They provide double stabilization: steric and electrostatic one.
Their dispersing power is further enhanced if aromatic rings are present in the hydrophobic moiety of the surfactants: the bulky hydrophobic structure with four aromatic rings of tristyrylphenol ethoxylate sulphates guarantees excellent absorption on the solid to be dispersed.
Applications

Thanks to the combination of high dispersing power and high stabilizing properties, our sulfates surfactants show excellent performances in active ingredient dispersions for the agriculture sector (sulfates from tristyrylphenol ethoxylates) and in pigment dispersions for the textile and paint sector.
Emulsifying and stabilizing properties of our sulfates surfactants are highly appreciated in emulsion polymerization processes to successfully polymerize different types of monomers with very low residue formation and to prevent possible re-agglomeration of the final latex particles.
